Cytotoxicity of mitochondrial Complex I inhibitor rotenone: a complex interplay of cell death pathways
DOI:
https://doi.org/10.26124/bec:2022-0014Keywords:
rotenone, mitochondria, ferroptosis, reactive oxygen and nitrogen species, RONS, neurodegenerationAbstract
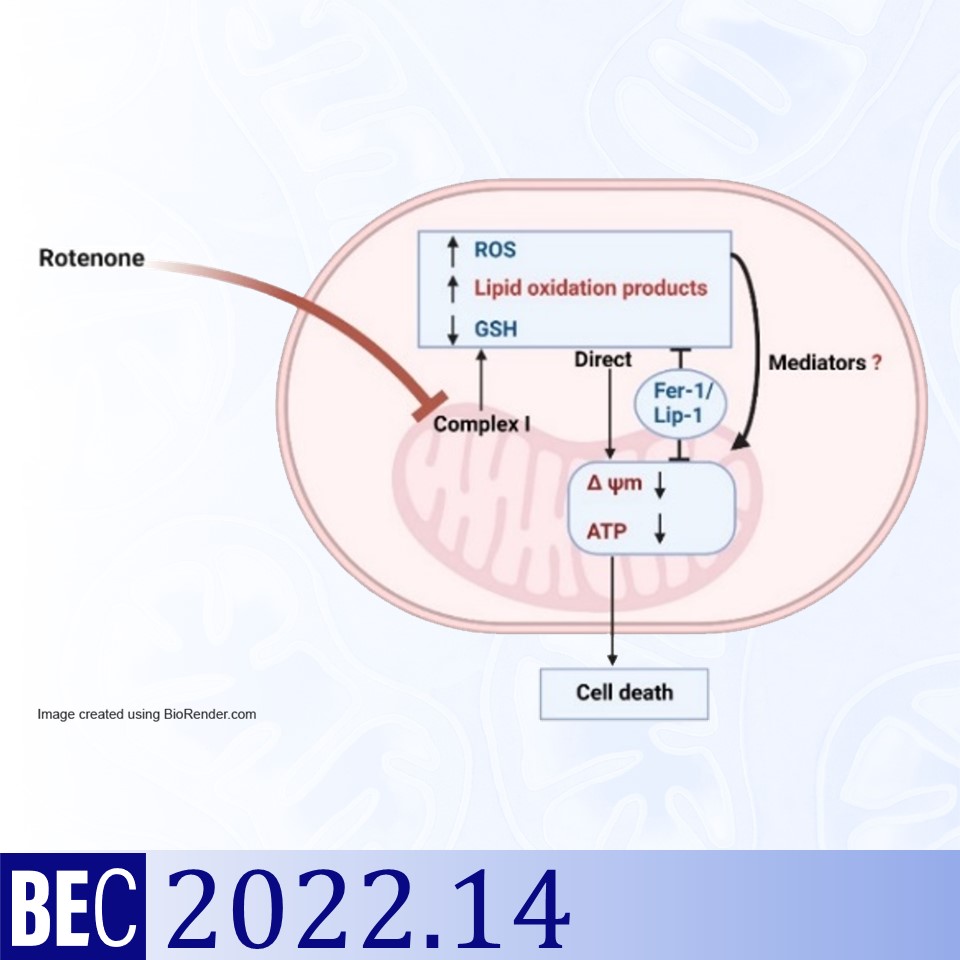
Ferroptosis, a type of regulated cell death, has been implicated in various diseases like cancer and neurodegenerative diseases including Parkinson’s disease. Because the mitochondrial Complex I inhibitor rotenone is widely used to develop experimental models of Parkinson’s disease, we examined if rotenone is an inducer of ferroptosis. Ferroptosis is iron-dependent and accompanied by increased production and accumulation of reactive oxygen and nitrogen species and lipid oxidation products, depletion of reduced glutathione, mitochondrial morphological alterations, and rupture of the plasma membrane. The mechanism of cell death in ferroptosis subsequent to the accumulation of ROS and lipid oxidation products, however, is not clearly established. We show here that rotenone (0.5 µM) causes the death of SH-SY5Y cells (a human neuroblastoma cell line) within 48 h accompanied by mitochondrial membrane depolarization, intracellular ATP depletion, increased production and accumulation of ROS and the lipid oxidation product malondialdehyde and a decrease in reduced glutathione content. These processes are inhibited conspicuously by novel inhibitors of ferroptosis such as ferrostatin-1 and liproxstatin-1 without rescuing the rotenone-induced inhibition of Complex I activity. Further, after rotenone-treatment both apoptotic and necrotic cells were noticed as evidenced from nuclear morphology after staining by Hoechst 33258 and propidium iodide, respectively. Taken together, rotenone was an inducer of ferroptosis in SH-SY5Y cells which presented morphological features of apoptosis and necrosis.
Cite:
Ganguly U, Bir A, Chakrabarti S (2022) Cytotoxicity of mitochondrial Complex I inhibitor rotenone: a complex interplay of cell death pathways. Bioenerg Commun 2022.14. https://doi.org/10.26124/bec:2022-0014
Downloads
Additional Files
Published
License
Copyright (c) 2022 Upasana Ganguly, Aritri Bir, Sasanka Chakrabarti

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.



